The Language of Stem Cell Medicine: What are They? What Makes Them so Special? And What do all Those Acronyms Mean?
Stem cell medicine is based on the concept that physicians can harness the body’s own reserves to heal itself, rather than relying exclusively on drugs or invasive surgical procedures. Stem cell medicine works by deals engineering human stem cells to replace or restore damaged or diseased organs or tissue, or establish normal function in them. While regenerative medicine primarily includes therapies a that utilize stem cells, the term is also used to describe therapies that use progenitor cells, used for many decades in the form of bone marrow transplants, as well as other cellular products such as platelet-rich plasma (PRP).
While both PRP and progenitor cells are widely used in clinical settings, stem cell therapies are still playing catch-up. PRP is used to treat orthopedic injuries and degenerative joint disease.
However, stem cells are in high demand worldwide. The burgeoning field of stem cell medicine is widely understood in a vague sort of way, but few people are aware that there are different kinds of stem cells. They can be derived from different tissue sources, harvested from the patient’s own body or donated. To help establish a better understanding of the stem cell landscape, we’ll start with some basic concepts.
Autologous vs. Allogenic Stem Cells
Stem cell treatments are generally divided into two classes:
- Autologous stem cells – collected from your own body, exclusively for your own use
- allogeneic stem cells, harvested from another person (donor)
Current clinical trials involving both autologous and allogeneic therapies are taking place all over the world. These trials target a wide range of diseases and conditions, from heart disease to orthopedic conditions, to wound healing.
Autologous treatments using your own stem cells can be performed in the same operative session, which eliminates concerns over your body rejecting donor cells. Your stem cells are extracted from your tissue, and reinjected back into your body targeting the area or organ that needs mending. This is a one-to-one therapy.
Allogeneic therapies use stem cells donated from another person. Before these cells can be put into a different human body than the one they came from, they must undergo extensive testing for diseases, and the cells are usually culture expanded in laboratories to achieve higher cell counts. Allogeneic therapies are performed under strict FDA guidelines, as these stem cells can eventually scale up in mass production, be stored and potentially distributed to millions of patients.
Stem Cell Types
 Adult stem cells (non-embryonic) are undifferentiated cells found throughout the body that multiply by cell division to replenish dying cells and regenerate damaged tissues.
Adult stem cells (non-embryonic) are undifferentiated cells found throughout the body that multiply by cell division to replenish dying cells and regenerate damaged tissues.
Stem cells are acquired from various tissue sources, and each tissue source has different potentials for the cells to differentiate. The following information explains these tissue sources and corresponding type of stem cells:
Adult Stem Cells (ASC’s)
In recent decades researchers discovered that stem cells can be found in all adult tissues. These are called adult stem cells, and although they cannot differentiate into every type of cell like embryonic stem cells, they can differentiate into bone, cartilage and adipose (fat) tissue readily. The two most familiar sources of adult stem cells are bone marrow and adipose tissue. More than 2,000 clinical trials have been conducted worldwide using the various tissue sources of adult stem cells.
IPS Cells (induced pluripotent cells)
IPS cells come from adult cells. Their genetic code is biologically manipulated to become pluripotent, which means they can differentiate, or become any other type of cell. Because the genetic code of IPS cells has been altered, they carry a higher risk profile than both adult stem cells and embryonic stem cells.
Embryonic Stem Cells (ES)
Embryonic stem cells, first isolated in mouse embryos in 1981, are derived from the embryo of a human fetus. Controversy has pursued embryonic stem cell research since its inception, over of ethical and religious perceptions. Embryonic stem cells are currently used mainly for research and understanding how regenerative cells work.
Types of Adult Stem Cells
Adult stem cells can be isolated from bone marrow, adipose tissue, umbilical cord blood, peripheral blood, dental pump, and  other sources. Most recently, a large number of clinical trials are focusing on stem cells derived from bone marrow and adipose tissue.
other sources. Most recently, a large number of clinical trials are focusing on stem cells derived from bone marrow and adipose tissue.
Bone Marrow Stem Cells
Bone marrow stem cells were the first recognized form of adult stem cells in the body. Researchers found they could be used to help heal bone and to replace different cell types in the blood. They could also be used in cancer patients whose bone marrow was destroyed by radiation therapy or chemotherapy. Use of bone marrow stem cells is FDA approved under certain conditions.
The drawback with bone marrow stem cells is that they are difficult to extract and not abundant. In order to be used as a treatment, bone marrow stem cells must be expanded in culture in a lab. The FDA places this therapy in the category of a drug, and requires rigorous oversight and testing.
Adipose Derived Stem Cells
In 2001, researchers and plastic surgeons from the University of Pittsburgh discovered that human fat tissue is a very rich source of mesenchymal stem cells (MSCs), multipotent stromal cells that can differentiate into a variety of cell types, and the findings were published in Tissue Engineering Journal. Upon publication, this discovery stirred quite an epiphany in the medical and scientific community—until then, adult MSCs were predominantly believed to be strictly a bone marrow product.
###

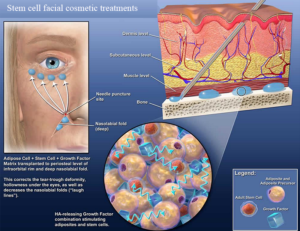
 Over the past 10 years, plastic surgeons have established safe and convenient ways to remove fat and isolate the stem cells for use in cosmetic procedures. And since adipose stem cells are extracted and reintroduced to the patient’s own body, the risk of rejection that goes with donor stem cells is eliminated. Scores of ongoing clinical trials using adipose stem cells have already proven their safety and efficacy in a variety of applications. Anti-aging therapies using adipose stem cells, for instance, have grown exponentially in popularity.
Over the past 10 years, plastic surgeons have established safe and convenient ways to remove fat and isolate the stem cells for use in cosmetic procedures. And since adipose stem cells are extracted and reintroduced to the patient’s own body, the risk of rejection that goes with donor stem cells is eliminated. Scores of ongoing clinical trials using adipose stem cells have already proven their safety and efficacy in a variety of applications. Anti-aging therapies using adipose stem cells, for instance, have grown exponentially in popularity.
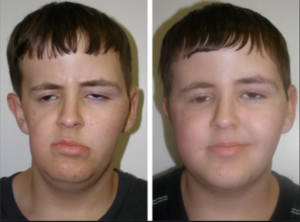
 taking on a new challenge—unifying the powerful hospitals that train Harvard’s medical students.
taking on a new challenge—unifying the powerful hospitals that train Harvard’s medical students.

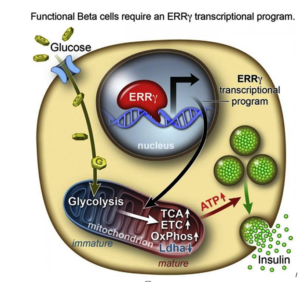
 After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
 s because it’s so important in learning, memory and mood function,” Shetty said. “We’re interested in understanding aging in the brain, especially in the hippocampus, which seems particularly vulnerable to age-related changes.”
s because it’s so important in learning, memory and mood function,” Shetty said. “We’re interested in understanding aging in the brain, especially in the hippocampus, which seems particularly vulnerable to age-related changes.” Shetty’s previous research focused on the benefits of resveratrol (an antioxidant that is famously found in red wine and the skin of red grapes, as well as in peanuts and some berries) to the hippocampus. Although the results indicated important benefits for preventing memory loss in aging brains, his newest work demonstrates a way to affect the function of the hippocampus more directly.
Shetty’s previous research focused on the benefits of resveratrol (an antioxidant that is famously found in red wine and the skin of red grapes, as well as in peanuts and some berries) to the hippocampus. Although the results indicated important benefits for preventing memory loss in aging brains, his newest work demonstrates a way to affect the function of the hippocampus more directly.
 International Society for Stem Cell Research (ISSCR) is the largest professional organization of stem cell scientists. In 2007, ISSCR impaneled a broad international taskforce to develop a set of professional guidelines for responsible
International Society for Stem Cell Research (ISSCR) is the largest professional organization of stem cell scientists. In 2007, ISSCR impaneled a broad international taskforce to develop a set of professional guidelines for responsible