Researchers Move Closer to Lung Stem Cell Therapies to Treat Chronic Lung Diseases
Chronic lung diseases are the third leading causes of death in the U.S. Chronic lung diseases include a collection of illnesses that cause airflow blockage and breathing-related issues, including primarily chronic obstructive pulmonary disease (COPD), bronchitis, emphysema and asthma. Lung disease involves changes in cells within the lungs, and while research on lung stem cell therapies may not only shed light on their causes, it may provide the groundwork for future treatments.
Stem cells in the lung
Human lungs are hard working organs. In an average lifetime, human lungs take 20-40 million breaths and experience a daily airflow of between 1,850 and 2,640 gallons. Human lungs are made up of two distinct regions:
- The conducting airway tubes, including the trachea, bronchi, and bronchioles.
- The gas exchange regions, or alveolar spaces.
Medical researchers have discovered that these regions each contain unique types of stem cells and progenitor cells. In normal lungs, an abundance of progenitor cells is present in each region, which divide to replace old or damaged lung cells to keep the lungs healthy. The progenitor cells include tracheal basal cells, bronchiolar secretory cells (known as club cells), and alveolar type 2 cells. Progenitor cell division is believed to be sufficient to renew the lung’s structure throughout normal adult life.
Stem cells are far less abundant than progenitors, but are found in both embryonic and adult lungs. Some stem cells assist in initial lung development, while others help repair and regenerate the lung throughout one’s lifetime. Problematic stem cells may actually contribute to lung diseases. In mouse lungs, certain rare stem cells have been located in the conducting airway tubes after to severe injury—for example, flu infection. These rare cells can divide and produce new cells that contribute to both the airway and gas exchange regions. These cells have also been grown in vitro and used as a proof-of-concept treatment in injured mouse lungs.
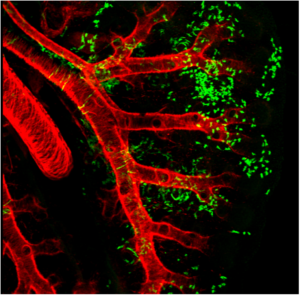
Wnt2+ CPPs (green cells) populate multiple cell lineages in the developing lung including airway and vascular smooth muscle. The smooth muscle of the branching airways and large blood vessels are stained in red.
Adult mesenchymal stem cells (hMSCs)
Adult human mesenchymal stem cells (hMSCs) are the focus of a number of clinical applications. The advantage of hMSCs is that they are immuno-modulatory— capable of modifying or regulating one or more immune functions—and versatile due to the anti-inflammatory and regenerative bioactive molecules they secrete.
hMSCs have the potential to orchestrate reparative processes in diseased or injured tissues. Much of the diversity and uniqueness of hMSCs is defined by their response to the environment of injured tissue. hMSCs are sensitive to their site-specific microenvironment, and scientists anticipate that these cells will deliver the bioactive agents in a site-specific manner quite different from the way pharmaceutical drugs work in the treatment of lung diseases.
hMSCs are non-hematopoietic, multi-potent progenitor cells with the capacity to generate bone marrow stromal cells as well as adipocytes, chondrocytes, and osteocytes in suitable tissue and other organ sites.
Studying lung stem cells sheds light on the causes of lung disease
A better understanding of lung stem cell and progenitor cell biology can improve our knowledge of how the healthy lung works. This in turn will shed light on the causes of lung diseases such as chronic obstructive pulmonary disease (COPD). Such research could lead to the development of new treatments for lung disease. In fact, lung stem cells may be used in future therapies to repair or regenerate the lungs of patients with severe lung damage or disease.
Current research
Lung stem cells have most frequently been identified and characterized in mice. Studies on mice have allowed researchers to identify the differences between embryonic and adult lung stem cells, discover the role of stem cells in lung repair, and investigate how changes to lung stem cells may lead to lung disease. A current focus of research includes testing if the same stem and progenitor cell populations can be identified in human lungs.
repair, and investigate how changes to lung stem cells may lead to lung disease. A current focus of research includes testing if the same stem and progenitor cell populations can be identified in human lungs.
Identifying progenitor and stem cells before and after lung injury
Researchers are also working to determine the role of stem cells in various human lung diseases, including lung cancer and COPD. They have begun examining potential clinical applications of stem cell therapies with several ‘first-in-human’ studies to investigate whether lung stem cells might enhance organ replacement or regeneration in patients.
The future of stem cells in treating lung disease
As researchers continue to improve their understanding of the exact identity and function of human lung stem cells, the potential for clinical applications will be divulged. Researchers will identify methods to control lung stem cells, which can then be tested as treatments for lung diseases. Further research will also investigate the uses of lung stem cells for personalized medicine.
###

 blood vessels, skeletal muscle, skin, teeth, heart, gut, liver, ovarian epithelium, and testis. They are thought to reside in a specific area of each tissue (called a “stem cell niche”). In many tissues, current evidence suggests that some types of stem cells are pericytes, cells that compose the outermost layer of small blood vessels. Stem cells may remain quiescent (non-dividing) for long periods of time until they are activated by a normal need for more cells to maintain tissues, or by disease or tissue injury.
blood vessels, skeletal muscle, skin, teeth, heart, gut, liver, ovarian epithelium, and testis. They are thought to reside in a specific area of each tissue (called a “stem cell niche”). In many tissues, current evidence suggests that some types of stem cells are pericytes, cells that compose the outermost layer of small blood vessels. Stem cells may remain quiescent (non-dividing) for long periods of time until they are activated by a normal need for more cells to maintain tissues, or by disease or tissue injury.
 In the study, scientists investigated the role of the
In the study, scientists investigated the role of the  cone cells, which now can be produced in high quantities.
cone cells, which now can be produced in high quantities. “The goal isn’t just to make the closest thing next to a real retina, but also to possibly harness the flexibility of the system to create more diverse ways of studying retina tissue,” says senior author Mike Karl, of the German Center for Neurodegenerative Diseases (DZNE) and part of the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden.
“The goal isn’t just to make the closest thing next to a real retina, but also to possibly harness the flexibility of the system to create more diverse ways of studying retina tissue,” says senior author Mike Karl, of the German Center for Neurodegenerative Diseases (DZNE) and part of the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden.