Stem Cell Treatments Normally Used for Cancer Patients are Helping Multiple Sclerosis Patients
The British Broadcasting Corporation (BBC) recently reported that stem cell transplant treatments normally used for cancer patients are helping Multiple Sclerosis (MS) patients in the UK. According to the January 18, 2016 report, 20 patients received bone marrow stem cell transplants using their own stem cells, and that at least some of the patients who were paralyzed by MS are able to walk again post-treatment.
Approximately100,000 people in the United Kingdom suffer from MS, with most new patients diagnosed between the ages of 20 and 30 years of age.
“To have a treatment which can potentially reverse disability is really a major achievement,” says Prof Basil Sharrack, of Sheffield’s Royal Hallamshire Hospital in Sheffield, England.
The treatment, known as autologous hematopoietic stem cell transplantation (HSCT), involves the intravenous infusion of autologous or allogeneic stem cells harvested from the patient’s own bone marrow to reestablish hematopoietic function (formation of blood or blood cells) in patients whose bone marrow or immune system is damaged or defective by chemotherapy. Using stem cells harvested from the patient’s bone marrow helps rebuild the immune system. The theory is that these newly harvested cells are at such an early stage in development that the cellular defects that result in MS do not exist.
“The immune system is being reset or rebooted back to a time point before it caused MS,” says Prof John Snowden, consultant hematologist at Royal Hallamshire Hospital.
The BBC’s Panorama program spoke to several MS patients who have undergone the stem cell transplant.
Steven Storey was diagnosed with MS in 2013 and, within a year, went from being an able-bodied athlete to wheelchair dependent and losing sensation in much of his body.
“I went from running marathons to needing 24-hour acute care. At one point I couldn’t even hold a spoon and feed myself,” Storey says.
Within a few days of the transplant Storey was able to move his toes, and after four months he could stand unaided.
While Storey still needs a wheelchair for mobility, he calls his progress astounding.
“It’s been incredible,” he says. “I was in a dire place, but now I can swim and cycle and I am determined to walk.”
The Royal Hallamshire Hospital along with hospitals in the United States, Sweden and Brazil, is part of an international clinical trial called MIST that is assessing the long-term benefits of the stem cell procedure on MS patients. Study participants all have relapsing remitting MS (RRMS), and received intensive chemotherapy to completely destroy the patients’ immune systems.
Treatment costs are about the same as the annual cost for existing treatments, and the stem cell treatment does not require the use of new or existing medications.
Prof Richard Burt of Northwestern University in Chicago carried out the first hematopoietic stem cell transplantation for MS in 1995, and is coordinating this current MIST international trial, which began in 2006.
“There has been resistance to this in the pharma and academic world,” Burt says. “This is not a technology you can patent and we have achieved this without industry backing.”
A study published last year involving MS patients in Chicago showed significant reductions in neurological disability, and for some the improvements persisted for at least four years, although there was no comparative control group.
The outcomes of the current international trial will be reported in 2018, and may determine whether the stem cell transplant becomes a standard in the United Kingdoms health care system for many MS patients.
“Ongoing research suggests stem cell treatments such as HSCT could offer hope, and it’s clear that in the cases highlighted by Panorama they’ve had a life-changing impact,” says Emma Gray, M.D., head of clinical trials at UK’s MS Society.

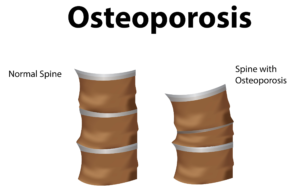
 porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
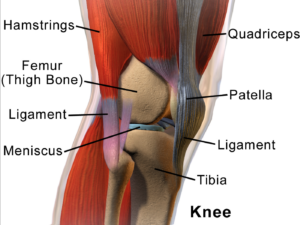
porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly. n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and
n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and  muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon. Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both. dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.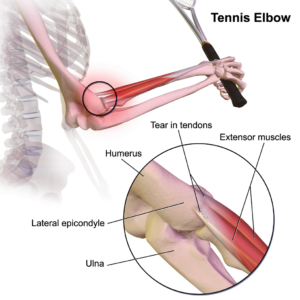
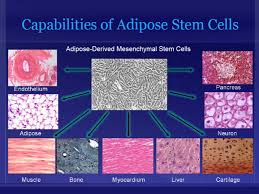
 themselves, they can divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions. MSCs are attractive to researchers and clinicians since they can be readily isolated from a variety of patient tissues, including fat. Once harvested and, if necessary, elevated to high numbers in culture, MSCs can be re-introduced to the patient from whom they were harvested, eliminating any risk of immune-rejection. In response to injury, MSCs produce proteins that appear to alter the surrounding environment and promote healing and tissue regeneration, such as anti-inflammatory factors, angiogenic factors (which promote the growth of new blood vessels) and other factors that stimulate local, tissue-specific stem cells.
themselves, they can divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions. MSCs are attractive to researchers and clinicians since they can be readily isolated from a variety of patient tissues, including fat. Once harvested and, if necessary, elevated to high numbers in culture, MSCs can be re-introduced to the patient from whom they were harvested, eliminating any risk of immune-rejection. In response to injury, MSCs produce proteins that appear to alter the surrounding environment and promote healing and tissue regeneration, such as anti-inflammatory factors, angiogenic factors (which promote the growth of new blood vessels) and other factors that stimulate local, tissue-specific stem cells. one study using the St. George Respiratory Questionnaire—a disease-specific tool designed to measure impact on overall health, daily life, and perceived well-being in patients with obstructive airways disease—we found that patients demonstrate statistically significant improvements post-treatment, out to the six month time period, including improvements in their ability to complete the six-minute walk distance or exercise capacity.
one study using the St. George Respiratory Questionnaire—a disease-specific tool designed to measure impact on overall health, daily life, and perceived well-being in patients with obstructive airways disease—we found that patients demonstrate statistically significant improvements post-treatment, out to the six month time period, including improvements in their ability to complete the six-minute walk distance or exercise capacity. Another very promising trial Global Stem Cells Group completed for
Another very promising trial Global Stem Cells Group completed for  Take, for instance, breast augmentation using this process. By taking fat from one location, relocating it, and adding stem cells with the fat to the breast tissue, you can reduce reabsorption of the fat tissue. In addition to being able to perform fat transfer for breast augmentation, you can also utilize the stem cells and platelet-rich plasma (PRP) when you need to rejuvenate the skin as well. One example would be a patient who had received an injection of stem cells plus platelet rich plasma without a fat graft: in this case, the cell will be very angiogenic in nature, creating new blood vessels and generating a youthful glow. The cells can also help with collagen production so the patient gets smoother skin and help with scarring or the appearance of unevenness on the skin.
Take, for instance, breast augmentation using this process. By taking fat from one location, relocating it, and adding stem cells with the fat to the breast tissue, you can reduce reabsorption of the fat tissue. In addition to being able to perform fat transfer for breast augmentation, you can also utilize the stem cells and platelet-rich plasma (PRP) when you need to rejuvenate the skin as well. One example would be a patient who had received an injection of stem cells plus platelet rich plasma without a fat graft: in this case, the cell will be very angiogenic in nature, creating new blood vessels and generating a youthful glow. The cells can also help with collagen production so the patient gets smoother skin and help with scarring or the appearance of unevenness on the skin. Again, courtesy of Dr. Purita, is another example of a patient with
Again, courtesy of Dr. Purita, is another example of a patient with 

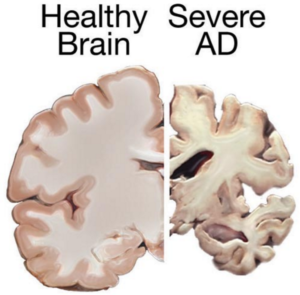 cognitive and behavioral symptoms, but have little to no effect on the disease’s development over the long term. Current medications can’t stop Alzheimer’s from progressing, but they can temporary lessen symptoms like confusion and memory loss.
cognitive and behavioral symptoms, but have little to no effect on the disease’s development over the long term. Current medications can’t stop Alzheimer’s from progressing, but they can temporary lessen symptoms like confusion and memory loss. A different approach would be to use stem cells for delivering
A different approach would be to use stem cells for delivering