Could stem cells repair the damaged brain in Alzheimer’s?
Stem cell therapies may hold the cure to Alzheimer’s, although so far that cure has been elusive. People who suffer from Alzheimer’s disease experience disorientation regarding time and place, changes in mood, personality and behavior, memory loss, difficulty solving problems or planning, and difficulty writing or performing other routine and familiar tasks. This progressive and irreversible brain disorder may affect judgment, initiative and social life, and can lead to physical symptoms such as vision problems.
Alzheimer’s affects mostly people aged 70 years and above, and is more common in women. It is the main risk factor for dementia among the elderly.
There is no known cure for Alzheimer’s. Conventional treatments, both drug-based and non-drug strategies, may help with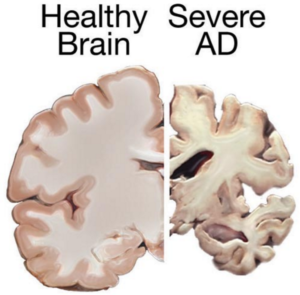 cognitive and behavioral symptoms, but have little to no effect on the disease’s development over the long term. Current medications can’t stop Alzheimer’s from progressing, but they can temporary lessen symptoms like confusion and memory loss.
cognitive and behavioral symptoms, but have little to no effect on the disease’s development over the long term. Current medications can’t stop Alzheimer’s from progressing, but they can temporary lessen symptoms like confusion and memory loss.
Although there have been attempts to find a remedy for Alzheimer’s, and despite the fact that scientists have managed to effectively treat lab animals with drug-based treatments, no animal model has managed to truly mimic its symptoms as they manifest in humans. Remedies that worked in lab animals have failed to work in humans; for this reason, scientists decided to try a different approach by exploring the possibilities of stem cells therapies in Alzheimer’s treatments.
Can Stem cells develop new Alzheimer’s treatments?
Alzheimer’s disease affects neurons in all parts of the brain, and the complexity of this condition makes it difficult to create a model that perfectly mimics its manifestations. At least in theory, stem cells could be used for treating this condition by transplanting neural stem cells into the patient’s brain in an attempt to generate healthy new neurons to replace dead and damaged neurons. It remains unclear whether the brain would be able to integrate the transplanted cells, and if the neural stem cells are able to travel to the damaged areas.
Another great challenge is producing the different types of neurons needed to replace the damaged cells, and to find a way to stimulate the renewal of the lost connections between neural cells. Even if the transplanted cells survive and find their way to the damaged areas, they might become damaged by proteins that build up in the brain—the same proteins that cause the disease in the first place, which means any effects of a stem cell transplant could be only temporary.
 A different approach would be to use stem cells for delivering neurotrophins to the brain. Neurotrophin proteins support the growth and survival of neurons, but in patients with Alzheimer’s, they’re produced in amounts too low to contribute such support. Neural stem cells can produce such cells, and in mice tests this method did prove helpful; scientists observed some improvements in memory in mice treated with stem cells.
A different approach would be to use stem cells for delivering neurotrophins to the brain. Neurotrophin proteins support the growth and survival of neurons, but in patients with Alzheimer’s, they’re produced in amounts too low to contribute such support. Neural stem cells can produce such cells, and in mice tests this method did prove helpful; scientists observed some improvements in memory in mice treated with stem cells.
Mesenchymal stem cells could also be used for treating Alzheimer’s—not to replace damaged neurons, but to heal them. Mesenchymal stem cells may exert anti-inflammatory effects and may help ameliorate the symptoms of Alzheimer’s, but there’s no study at the moment to prove their safety or effectiveness in this condition [3].
Although clinical trials and studies on Alzheimer’s disease treatment to date have a high failure rate, the use of stem cells may be helpful for studying the behavior of brain cells damaged by the condition, as well as for testing various therapeutic approaches and predicting which treatment may help Alzheimer’s patients.
Researchers from the Harvard Stem Cells Institute took skin cells from Alzheimer’s patients and reprogramed them to create induced pluripotent stem cells (iPSC); obtained cells were grown in special lab conditions and were found to release the same proteins that form plaque in Alzheimer’s patients [2]. This may give scientists the opportunity to study the behavior of Alzheimer’s-affected cells and to search for and test new remedies.
Asian scientists managed to turn human fibroblasts into neuronal cells using a chemical cocktail of small molecules [6]. These findings may provide an alternative strategy for modeling the neurodegenerative disorder, which may help scientists understand the mechanisms behind this condition. It may also play an important role in identifying new stem cell based treatments.
References
- http://www.eurostemcell.org/factsheet/alzheimer%E2%80%99s-disease-how-could-stem-cells-help
- http://hsci.harvard.edu/news/alzheimer%E2%80%99s-dish
- http://www.ipscell.com/2012/05/can-stem-cells-be-used-to-treat-alzheimers-disease/
- http://www.sciencedirect.com/science/article/pii/S1934590915003173
- http://www.cell.com/cell-stem-cell/abstract/S1934-5909%2815%2900305-7

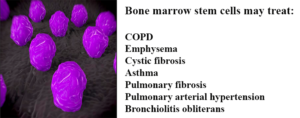 Acknowledging the similarities between lung and bone marrow stem cells, Professor Yair Reisner of the Immunology Department of the Weizmann Institute tested the ability of lung stem cells to travel to a specific region after transplantation in mice [1]. Before introducing the bone marrow stem cells into mouse models with lung damage, the group of scientists cleared the lungs’ stem cell compartments to clear a path for the transplanted cells.
Acknowledging the similarities between lung and bone marrow stem cells, Professor Yair Reisner of the Immunology Department of the Weizmann Institute tested the ability of lung stem cells to travel to a specific region after transplantation in mice [1]. Before introducing the bone marrow stem cells into mouse models with lung damage, the group of scientists cleared the lungs’ stem cell compartments to clear a path for the transplanted cells.
 Stem cells offer great potential for use in clinical applications thanks to their ability to specialize into different cell types and to renew themselves. Although some of them have limitations, stem cells are still an amazing resource for the medical world, as no other cell inside the human body has the ability to generate new cell types with a more specific function that the source.
Stem cells offer great potential for use in clinical applications thanks to their ability to specialize into different cell types and to renew themselves. Although some of them have limitations, stem cells are still an amazing resource for the medical world, as no other cell inside the human body has the ability to generate new cell types with a more specific function that the source.